Page 6 - 3. Lesson notes - Ch-3 Covalent Bonding
P. 6
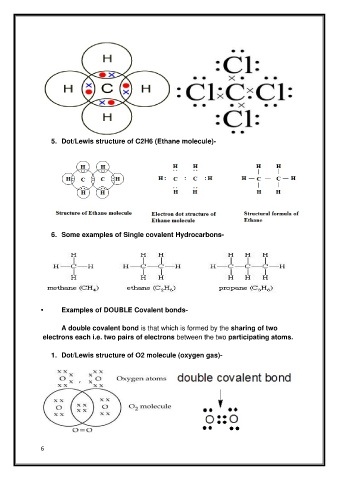
5. Dot/Lewis structure of C2H6 (Ethane molecule)-
6. Some examples of Single covalent Hydrocarbons-
• Examples of DOUBLE Covalent bonds-
A double covalent bond is that which is formed by the sharing of two
electrons each i.e. two pairs of electrons between the two participating atoms.
1. Dot/Lewis structure of O2 molecule (oxygen gas)-
6