Page 2 - 3. Lesson notes - Ch-3 Covalent Bonding
P. 2
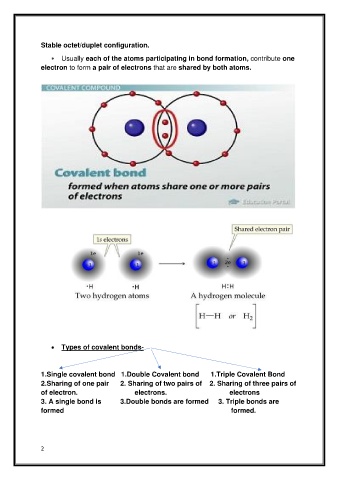
Stable octet/duplet configuration.
Usually each of the atoms participating in bond formation, contribute one
electron to form a pair of electrons that are shared by both atoms.
• Types of covalent bonds-
1.Single covalent bond 1.Double Covalent bond 1.Triple Covalent Bond
2.Sharing of one pair 2. Sharing of two pairs of 2. Sharing of three pairs of
of electron. electrons. electrons
3. A single bond is 3.Double bonds are formed 3. Triple bonds are
formed formed.
2