Page 9 - 3. Lesson notes - Ch-3 Covalent Bonding
P. 9
c. Covalent compounds do not conduct electricity in the aqueous or molten
state.
Reason- The absence of charged particles in covalent compounds is
responsible for the non-conductivity of electricity
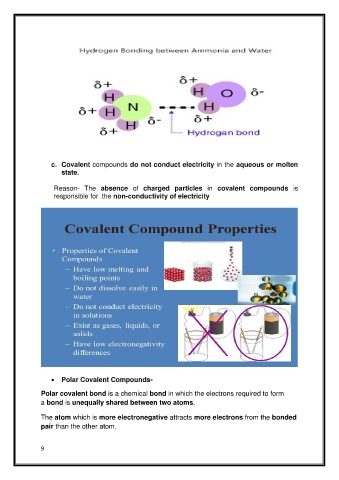
• Polar Covalent Compounds-
Polar covalent bond is a chemical bond in which the electrons required to form
a bond is unequally shared between two atoms.
The atom which is more electronegative attracts more electrons from the bonded
pair than the other atom.
9