Page 3 - LN
P. 3
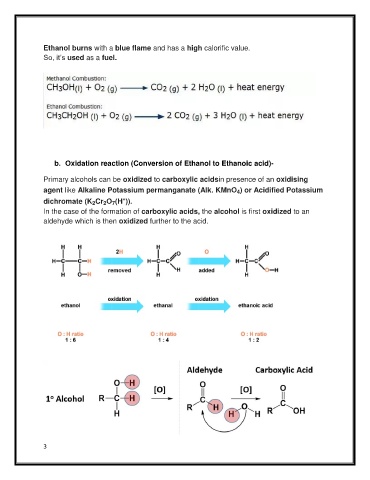
Ethanol burns with a blue flame and has a high calorific value.
So, it’s used as a fuel.
b. Oxidation reaction (Conversion of Ethanol to Ethanoic acid)-
Primary alcohols can be oxidized to carboxylic acidsin presence of an oxidising
agent like Alkaline Potassium permanganate (Alk. KMnO ) or Acidified Potassium
4
+
dichromate (K Cr O (H )).
2
2
7
In the case of the formation of carboxylic acids, the alcohol is first oxidized to an
aldehyde which is then oxidized further to the acid.
3