Page 1 - LN
P. 1
SAI International School
Class-X
Subject - Chemistry
Ch- Carbon & it's Compounds
Subtopic-Chemical Properties of Hydrocarbons
Lesson Notes
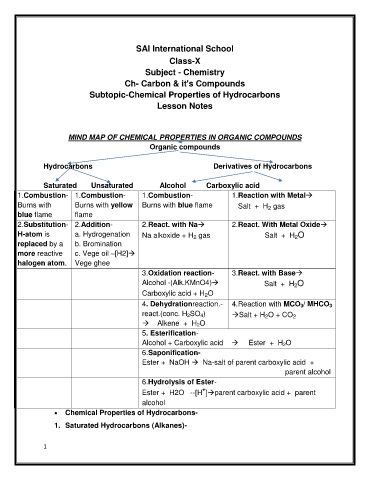
MIND MAP OF CHEMICAL PROPERTIES IN ORGANIC COMPOUNDS
Organic compounds
Hydrocarbons Derivatives of Hydrocarbons
Saturated Unsaturated Alcohol Carboxylic acid
1.Combustion- 1.Combustion- 1.Combustion- 1.Reaction with Metal
Burns with Burns with yellow Burns with blue flame Salt + H gas
2
blue flame flame
2.Substitution- 2.Addition- 2.React. with Na 2.React. With Metal Oxide
H-atom is a. Hydrogenation Na alkoxide + H gas Salt + H O
2
2
replaced by a b. Bromination
more reactive c. Vege oil –[H2]
halogen atom. Vege ghee
3.Oxidation reaction- 3.React. with Base
Alcohol -(Alk.KMnO4) Salt + H O
2
Carboxylic acid + H O
2
4. Dehydrationreaction.- 4.Reaction with MCO 3/ MHCO 3
react.(conc. H 2SO 4) Salt + H O + CO
2
2
Alkene + H 2O
5. Esterification-
Alcohol + Carboxylic acid Ester + H 2O
6.Saponification-
Ester + NaOH Na-salt of parent carboxylic acid +
parent alcohol
6.Hydrolysis of Ester-
+
Ester + H2O --[H ]parent carboxylic acid + parent
alcohol
Chemical Properties of Hydrocarbons-
1. Saturated Hydrocarbons (Alkanes)-
1