Page 5 - LN
P. 5
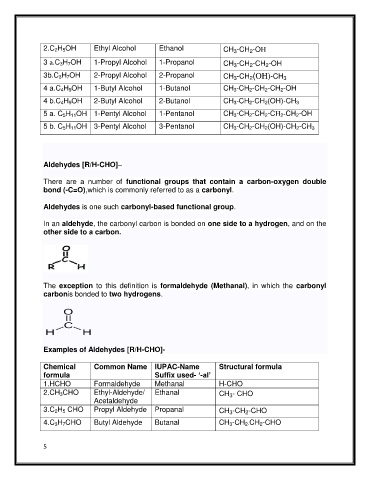
2.C 2H 5OH Ethyl Alcohol Ethanol CH -CH -OH
2
3
3 a.C 3H 7OH 1-Propyl Alcohol 1-Propanol CH -CH -CH -OH
3
2
2
3b.C 3H 7OH 2-Propyl Alcohol 2-Propanol CH -CH (OH)-CH
3
2
3
4 a.C 4H 9OH 1-Butyl Alcohol 1-Butanol CH 3-CH 2-CH 2-CH 2-OH
4 b.C 4H 9OH 2-Butyl Alcohol 2-Butanol CH 3-CH 2-CH 2(OH)-CH 3
5 a. C 5H 11OH 1-Pentyl Alcohol 1-Pentanol CH 3-CH 2-CH 2-CH 2-CH 2-OH
5 b. C 5H 11OH 3-Pentyl Alcohol 3-Pentanol CH 3-CH 2-CH 2(OH)-CH 2-CH 3
Aldehydes [R/H-CHO]–
There are a number of functional groups that contain a carbon-oxygen double
bond (-C=O),which is commonly referred to as a carbonyl.
Aldehydes is one such carbonyl-based functional group.
In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the
other side to a carbon.
The exception to this definition is formaldehyde (Methanal), in which the carbonyl
carbonis bonded to two hydrogens.
Examples of Aldehydes [R/H-CHO]-
Chemical Common Name IUPAC-Name Structural formula
formula Suffix used- ‘-al’
1.HCHO Formaldehyde Methanal H-CHO
2.CH 3CHO Ethyl-Aldehyde/ Ethanal CH - CHO
3
Acetaldehyde
3.C 2H 5 CHO Propyl Aldehyde Propanal CH -CH -CHO
2
3
4.C 3H 7CHO Butyl Aldehyde Butanal CH 3-CH 2-CH 2-CHO
5