Page 7 - LN
P. 7
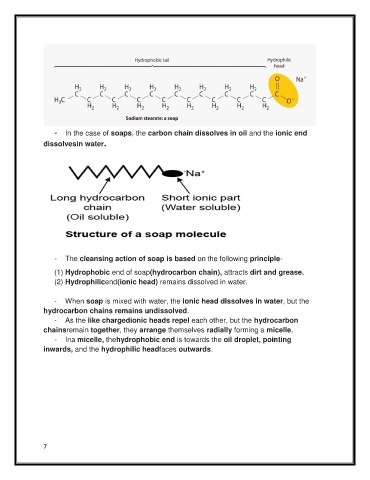
- In the case of soaps, the carbon chain dissolves in oil and the ionic end
dissolvesin water.
- The cleansing action of soap is based on the following principle-
(1) Hydrophobic end of soap(hydrocarbon chain), attracts dirt and grease.
(2) Hydrophilicend(ionic head) remains dissolved in water.
- When soap is mixed with water, the ionic head dissolves in water, but the
hydrocarbon chains remains undissolved.
- As the like chargedionic heads repel each other, but the hydrocarbon
chainsremain together, they arrange themselves radially forming a micelle,
- Ina micelle, thehydrophobic end is towards the oil droplet, pointing
inwards, and the hydrophilic headfaces outwards.
7