Page 10 - LN
P. 10
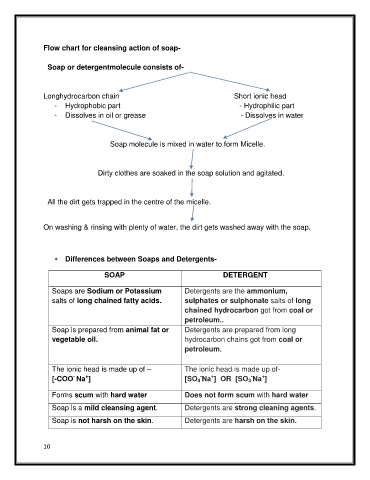
Flow chart for cleansing action of soap-
Soap or detergentmolecule consists of-
Longhydrocarbon chain Short ionic head
- Hydrophobic part - Hydrophilic part
- Dissolves in oil or grease - Dissolves in water
Soap molecule is mixed in water to form Micelle.
Dirty clothes are soaked in the soap solution and agitated.
All the dirt gets trapped in the centre of the micelle.
On washing & rinsing with plenty of water, the dirt gets washed away with the soap.
Differences between Soaps and Detergents-
SOAP DETERGENT
Soaps are Sodium or Potassium Detergents are the ammonium,
salts of long chained fatty acids. sulphates or sulphonate salts of long
chained hydrocarbon got from coal or
petroleum..
Soap is prepared from animal fat or Detergents are prepared from long
vegetable oil. hydrocarbon chains got from coal or
petroleum.
The ionic head is made up of – The ionic head is made up of-
+
-
+
-
+
-
[-COO Na ] [SO 4 Na ] OR [SO 3 Na ]
Forms scum with hard water Does not form scum with hard water
Soap is a mild cleansing agent. Detergents are strong cleaning agents.
Soap is not harsh on the skin. Detergents are harsh on the skin.
10