Page 2 - LN
P. 2
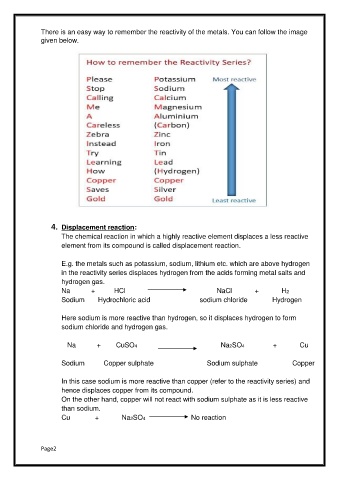
There is an easy way to remember the reactivity of the metals. You can follow the image
given below.
4. Displacement reaction:
The chemical reaction in which a highly reactive element displaces a less reactive
element from its compound is called displacement reaction.
E.g. the metals such as potassium, sodium, lithium etc. which are above hydrogen
in the reactivity series displaces hydrogen from the acids forming metal salts and
hydrogen gas.
Na + HCl NaCl + H2
Sodium Hydrochloric acid sodium chloride Hydrogen
Here sodium is more reactive than hydrogen, so it displaces hydrogen to form
sodium chloride and hydrogen gas.
Na + CuSO4 Na2SO4 + Cu
Sodium Copper sulphate Sodium sulphate Copper
In this case sodium is more reactive than copper (refer to the reactivity series) and
hence displaces copper from its compound.
On the other hand, copper will not react with sodium sulphate as it is less reactive
than sodium.
Cu + Na2SO4 No reaction
Page2