Page 3 - Lesson Notes - Biomolecules 1
P. 3
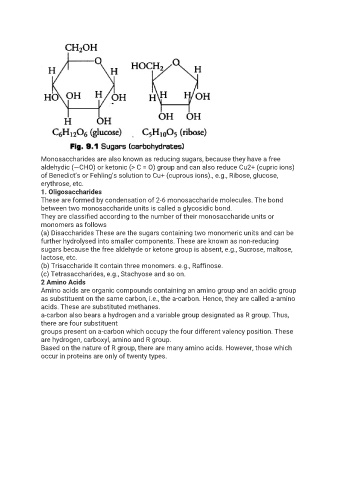
Monosaccharides are also known as reducing sugars, because they have a free
aldehydic (—CHO) or ketonic (> C = O) group and can also reduce Cu2+ (cupric ions)
of Benedict’s or Fehling’s solution to Cu+ (cuprous ions)., e.g., Ribose, glucose,
erythrose, etc.
1. Oligosaccharides
These are formed by condensation of 2-6 monosaccharide molecules. The bond
between two monosaccharide units is called a glycosidic bond.
They are classified according to the number of their monosaccharide units or
monomers as follows
(a) Disaccharides These are the sugars containing two monomeric units and can be
further hydrolysed into smaller components. These are known as non-reducing
sugars because the free aldehyde or ketone group is absent, e.g., Sucrose, maltose,
lactose, etc.
(b) Trisaccharide It contain three monomers. e.g., Raffinose.
(c) Tetrasaccharides, e.g., Stachyose and so on.
2 Amino Acids
Amino acids are organic compounds containing an amino group and an acidic group
as substituent on the same carbon, i.e., the a-carbon. Hence, they are called a-amino
acids. These are substituted methanes.
a-carbon also bears a hydrogen and a variable group designated as R group. Thus,
there are four substituent
groups present on a-carbon which occupy the four different valency position. These
are hydrogen, carboxyl, amino and R group.
Based on the nature of R group, there are many amino acids. However, those which
occur in proteins are only of twenty types.