Page 4 - 3. Lesson Note Ch-5 TRENDS IN THE PROPERTIES
P. 4
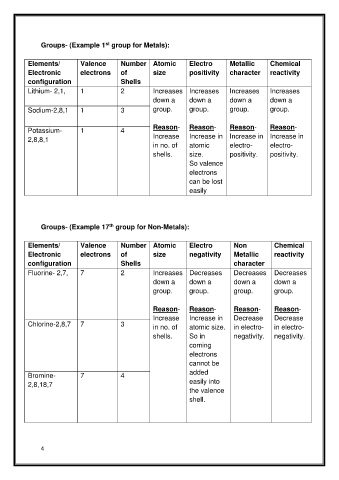
Groups- (Example 1 group for Metals):
st
Elements/ Valence Number Atomic Electro Metallic Chemical
Electronic electrons of size positivity character reactivity
configuration Shells
Lithium- 2,1, 1 2 Increases Increases Increases Increases
down a down a down a down a
Sodium-2,8,1 1 3 group. group. group. group.
Reason- Reason- Reason- Reason-
Potassium- 1 4
2,8,8,1 Increase Increase in Increase in Increase in
in no. of atomic electro- electro-
shells. size. positivity. positivity.
So valence
electrons
can be lost
easily
th
Groups- (Example 17 group for Non-Metals):
Elements/ Valence Number Atomic Electro Non Chemical
Electronic electrons of size negativity Metallic reactivity
configuration Shells character
Fluorine- 2,7, 7 2 Increases Decreases Decreases Decreases
down a down a down a down a
group. group. group. group.
Reason- Reason- Reason- Reason-
Increase Increase in Decrease Decrease
Chlorine-2,8,7 7 3 in no. of atomic size. in electro- in electro-
shells. So in negativity. negativity.
coming
electrons
cannot be
added
Bromine- 7 4
2,8,18,7 easily into
the valence
shell.
4