Page 3 - 3. Lesson Note Ch-5 TRENDS IN THE PROPERTIES
P. 3
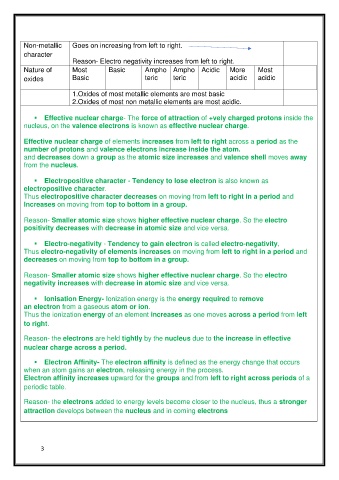
Non-metallic Goes on increasing from left to right.
character
Reason- Electro negativity increases from left to right.
Nature of Most Basic Ampho Ampho Acidic More Most
oxides Basic teric teric acidic acidic
1.Oxides of most metallic elements are most basic
2.Oxides of most non metallic elements are most acidic.
▪ Effective nuclear charge- The force of attraction of +vely charged protons inside the
nucleus, on the valence electrons is known as effective nuclear charge.
Effective nuclear charge of elements increases from left to right across a period as the
number of protons and valence electrons increase inside the atom.
and decreases down a group as the atomic size increases and valence shell moves away
from the nucleus.
▪ Electropositive character - Tendency to lose electron is also known as
electropositive character.
Thus electropositive character decreases on moving from left to right in a period and
Increases on moving from top to bottom in a group.
Reason- Smaller atomic size shows higher effective nuclear charge. So the electro
positivity decreases with decrease in atomic size and vice versa.
▪ Electro-negativity - Tendency to gain electron is called electro-negativity,
Thus electro-negativity of elements increases on moving from left to right in a period and
decreases on moving from top to bottom in a group.
Reason- Smaller atomic size shows higher effective nuclear charge. So the electro
negativity increases with decrease in atomic size and vice versa.
▪ Ionisation Energy- Ionization energy is the energy required to remove
an electron from a gaseous atom or ion.
Thus the ionization energy of an element increases as one moves across a period from left
to right.
Reason- the electrons are held tightly by the nucleus due to the increase in effective
nuclear charge across a period.
▪ Electron Affinity- The electron affinity is defined as the energy change that occurs
when an atom gains an electron, releasing energy in the process.
Electron affinity increases upward for the groups and from left to right across periods of a
periodic table.
Reason- the electrons added to energy levels become closer to the nucleus, thus a stronger
attraction develops between the nucleus and in coming electrons
3