Page 6 - 3. Lesson Note Ch-5 MODERN PERIODIC TABLE
P. 6
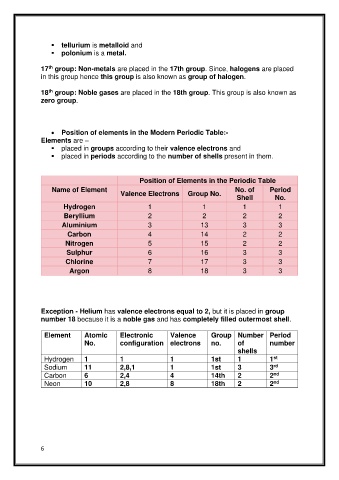
▪ tellurium is metalloid and
▪ polonium is a metal.
th
17 group: Non-metals are placed in the 17th group. Since, halogens are placed
in this group hence this group is also known as group of halogen.
th
18 group: Noble gases are placed in the 18th group. This group is also known as
zero group.
• Position of elements in the Modern Periodic Table:-
Elements are –
▪ placed in groups according to their valence electrons and
▪ placed in periods according to the number of shells present in them.
Position of Elements in the Periodic Table
Name of Element No. of Period
Valence Electrons Group No.
Shell No.
Hydrogen 1 1 1 1
Beryllium 2 2 2 2
Aluminium 3 13 3 3
Carbon 4 14 2 2
Nitrogen 5 15 2 2
Sulphur 6 16 3 3
Chlorine 7 17 3 3
Argon 8 18 3 3
Exception - Helium has valence electrons equal to 2, but it is placed in group
number 18 because it is a noble gas and has completely filled outermost shell.
Element Atomic Electronic Valence Group Number Period
No. configuration electrons no. of number
shells
Hydrogen 1 1 1 1st 1 1
st
Sodium 11 2,8,1 1 1st 3 3
rd
nd
Carbon 6 2,4 4 14th 2 2
nd
Neon 10 2,8 8 18th 2 2
6