Page 4 - 3. Lesson Note Ch-5 MODERN PERIODIC TABLE
P. 4
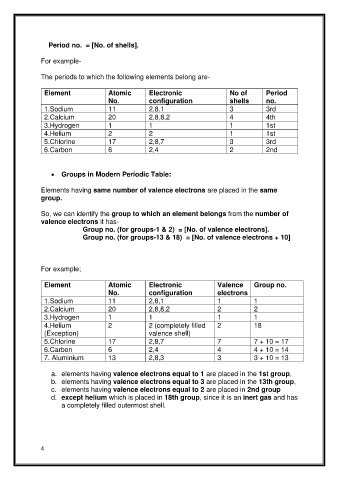
Period no. = [No. of shells].
For example-
The periods to which the following elements belong are-
Element Atomic Electronic No of Period
No. configuration shells no.
1.Sodium 11 2,8,1 3 3rd
2.Calcium 20 2,8,8,2 4 4th
3.Hydrogen 1 1 1 1st
4.Helium 2 2 1 1st
5.Chlorine 17 2,8,7 3 3rd
6.Carbon 6 2,4 2 2nd
• Groups in Modern Periodic Table:
Elements having same number of valence electrons are placed in the same
group.
So, we can identify the group to which an element belongs from the number of
valence electrons it has-
Group no. (for groups-1 & 2) = [No. of valence electrons].
Group no. (for groups-13 & 18) = [No. of valence electrons + 10]
For example;
Element Atomic Electronic Valence Group no.
No. configuration electrons
1.Sodium 11 2,8,1 1 1
2.Calcium 20 2,8,8,2 2 2
3.Hydrogen 1 1 1 1
4.Helium 2 2 (completely filled 2 18
(Exception) valence shell)
5.Chlorine 17 2,8,7 7 7 + 10 = 17
6.Carbon 6 2,4 4 4 + 10 = 14
7. Aluminium 13 2,8,3 3 3 + 10 = 13
a. elements having valence electrons equal to 1 are placed in the 1st group,
b. elements having valence electrons equal to 3 are placed in the 13th group,
c. elements having valence electrons equal to 2 are placed in 2nd group
d. except helium which is placed in 18th group, since it is an inert gas and has
a completely filled outermost shell.
4