Page 2 - 3.LESSON NOTES (CH-2 CONCEPTS OF ACIDS & BASES)
P. 2
(Arrhenius Concept of Acids & Bases)
ACIDS & BASES may be Classified as-
Strong and Weak Diluted and concentrated Basicity & acidity
1. Strong and Weak-
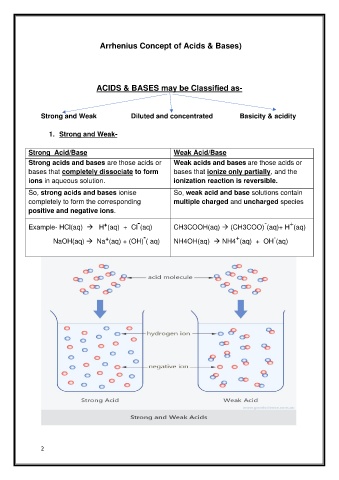
Strong Acid/Base Weak Acid/Base
Strong acids and bases are those acids or Weak acids and bases are those acids or
bases that completely dissociate to form bases that ionize only partially, and the
ions in aqueous solution. ionization reaction is reversible.
So, strong acids and bases ionise So, weak acid and base solutions contain
completely to form the corresponding multiple charged and uncharged species
positive and negative ions.
-
-
+
+
Example- HCl(aq) → H (aq) + Cl (aq) CH3COOH(aq) → (CH3COO) (aq)+ H (aq)
-
+
-
+
NaOH(aq) → Na (aq) + (OH) ( aq) NH4OH(aq) → NH4 (aq) + OH (aq)
2