Page 4 - LN
P. 4
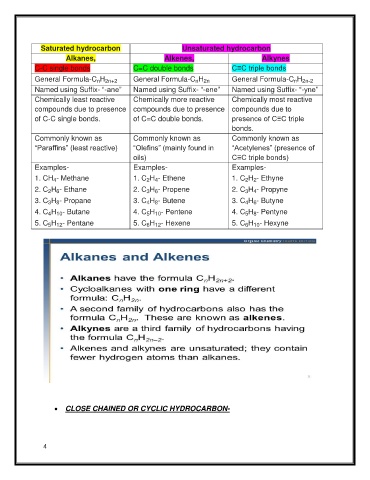
Saturated hydrocarbon Unsaturated hydrocarbon
Alkanes, Alkenes, Alkynes
C-C single bonds C=C double bonds C≡C triple bonds
General Formula-C H General Formula-C H General Formula-C H
n 2n
n 2n+2
n 2n-2
Named using Suffix- “-ane” Named using Suffix- “-ene” Named using Suffix- “-yne”
Chemically least reactive Chemically more reactive Chemically most reactive
compounds due to presence compounds due to presence compounds due to
of C-C single bonds. of C=C double bonds. presence of C≡C triple
bonds.
Commonly known as Commonly known as Commonly known as
“Paraffins” (least reactive) “Olefins” (mainly found in “Acetylenes” (presence of
oils) C≡C triple bonds)
Examples- Examples- Examples-
1
1
1. CH - Methane . C H - Ethene . C H - Ethyne
4
2 4
2 2
2
2. C H - Ethane 2. C H - Propene . C H - Propyne
3 4
2 6
3 6
3
3
3. C H - Propane C H - Butene . C H - Butyne
.
4 8
4 6
3 8
.
C
4
4. C H - Butane C H - Pentene H - Pentyne
.
4
5 8
4 10
5 10
5. C H - Pentane 5. C H - Hexene 5. C H - Hexyne
6 12
5 12
6 10
CLOSE CHAINED OR CYCLIC HYDROCARBON-
4