Page 4 - MM
P. 4
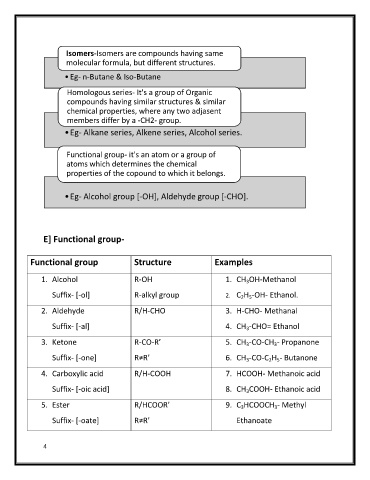
Isomers-Isomers are compounds having same
molecular formula, but different structures.
•Eg- n-Butane & Iso-Butane
Homologous series- It's a group of Organic
compounds having similar structures & similar
chemical properties, where any two adjasent
members differ by a -CH2- group.
•Eg- Alkane series, Alkene series, Alcohol series.
Functional group- it's an atom or a group of
atoms which determines the chemical
properties of the copound to which it belongs.
•Eg- Alcohol group [-OH], Aldehyde group [-CHO].
E] Functional group-
Functional group Structure Examples
1. Alcohol R-OH 1. CH OH-Methanol
3
Suffix- [-ol] R-alkyl group 2. C H -OH- Ethanol.
2 5
R
2. Aldehyde /H-CHO 3. H-CHO- Methanal
Suffix- [-al] 4. CH -CHO= Ethanol
3
-
R
3. Ketone CO-R’ 5. CH -CO-CH - Propanone
3
3
Suffix- [-one] R≠R’ 6. CH -CO-C H - Butanone
2 5
3
R
4. Carboxylic acid /H-COOH 7. HCOOH- Methanoic acid
Suffix- [-oic acid] 8. CH COOH- Ethanoic acid
3
5. Ester /HCOOR’ 9. C HCOOCH - Methyl
R
3
3
Suffix- [-oate] R≠R’ Ethanoate
4