Page 1 - MM
P. 1
SAI International School
Class-X
Subject_ Chemistry
Topic- Carbon & it's Compounds
Subtopic- Mind Map with concept mapping questions
[Revision of Carbon & its compounds]
MIND MAP
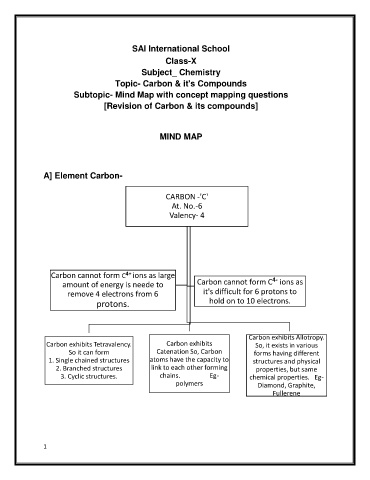
A] Element Carbon-
CARBON -'C'
At. No.-6
Valency- 4
4+
Carbon cannot form C ions as large 4-
amount of energy is neede to Carbon cannot form C ions as
remove 4 electrons from 6 it's difficult for 6 protons to
protons. hold on to 10 electrons.
Carbon exhibits Allotropy.
Carbon exhibits Tetravalency. Carbon exhibits So, it exists in various
Catenation So, Carbon
So it can form forms having different
1. Single chained structures atoms have the capacity to structures and physical
2. Branched structures link to each other forming properties, but same
3. Cyclic structures. chains. Eg- chemical properties. Eg-
polymers Diamond, Graphite,
Fullerene
1