Page 6 - 3. Lesson Notes - Ch-3 Mettalurgy-1
P. 6
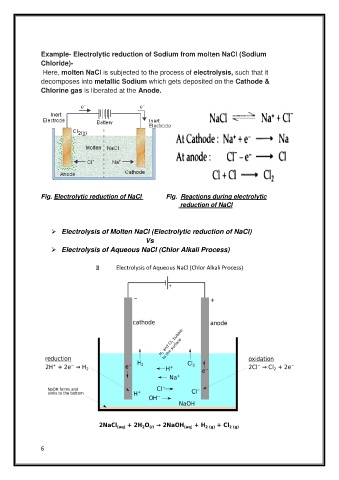
Example- Electrolytic reduction of Sodium from molten NaCl (Sodium
Chloride)-
Here, molten NaCl is subjected to the process of electrolysis, such that it
decomposes into metallic Sodium which gets deposited on the Cathode &
Chlorine gas is liberated at the Anode.
Fig. Electrolytic reduction of NaCl Fig. Reactions during electrolytic
reduction of NaCl
➢ Electrolysis of Molten NaCl (Electrolytic reduction of NaCl)
Vs
➢ Electrolysis of Aqueous NaCl (Chlor Alkali Process)
Electrolysis of Aqueous NaCl (Chlor Alkali Process)
6