Page 4 - 3. Lesson Notes - Ch-3 Mettalurgy-1
P. 4
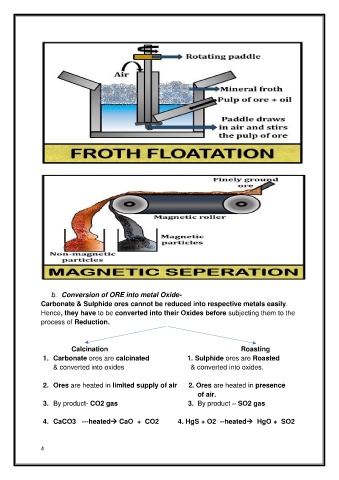
b. Conversion of ORE into metal Oxide-
Carbonate & Sulphide ores cannot be reduced into respective metals easily.
Hence, they have to be converted into their Oxides before subjecting them to the
process of Reduction.
Calcination Roasting
1. Carbonate ores are calcinated 1. Sulphide ores are Roasted
& converted into oxides & converted into oxides.
2. Ores are heated in limited supply of air 2. Ores are heated in presence
of air.
3. By product- CO2 gas 3. By product – SO2 gas
4. CaCO3 ---heated→ CaO + CO2 4. HgS + O2 --heated→ HgO + SO2
4