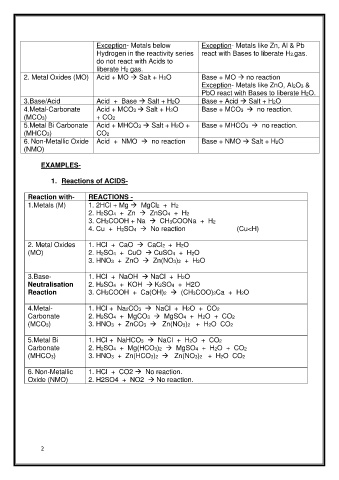
Page 2 - LN
P. 2
Exception- Metals below Exception- Metals like Zn, Al & Pb
Hydrogen in the reactivity series react with Bases to liberate H2.gas.
do not react with Acids to
liberate H2 gas.
2. Metal Oxides (MO) Acid + MO → Salt + H2O Base + MO → no reaction
Exception- Metals like ZnO, Al2O3 &
PbO react with Bases to liberate H2O.
3.Base/Acid Acid + Base → Salt + H2O Base + Acid → Salt + H2O
B
a
4.Metal-Carbonate Acid + MCO3 → Salt + H2O + MCO3 → no reaction.
se
(MCO3) + CO2
5.Metal Bi Carbonate Acid + MHCO3 → Salt + H2O + Base + MHCO3 → no reaction.
(MHCO3) CO2
6. Non-Metallic Oxide Acid + NMO → no reaction Base + NMO → Salt + H2O
(NMO)
EXAMPLES-
1. Reactions of ACIDS-
Reaction with- REACTIONS -
1.Metals (M) 1. 2HCl + Mg → MgCl2 + H2
2. H2SO4 + Zn → ZnSO4 + H2
3. CH3COOH + Na → CH3COONa + H2
4. Cu + H2SO4 → No reaction (Cu<H)
2. Metal Oxides 1. HCl + CaO → CaCl2 + H2O
(MO) 2. H2SO4 + CuO → CuSO4 + H2O
3. HNO3 + ZnO → Zn(NO3)2 + H2O
3.Base- 1. HCl + NaOH → NaCl + H2O
Neutralisation 2. H2SO4 + KOH → K2SO4 + H2O
Reaction 3. CH3COOH + Ca(OH)2 → (CH3COO)2Ca + H2O
4.Metal- 1. HCl + Na2CO3 → NaCl + H2O + CO2
Carbonate 2. H2SO4 + MgCO3 → MgSO4 + H2O + CO2
(MCO3) 3. HNO3 + ZnCO3 → Zn(NO3)2 + H2O CO2
5.Metal Bi 1. HCl + NaHCO3 → NaCl + H2O + CO2
Carbonate 2. H2SO4 + Mg(HCO3)2 → MgSO4 + H2O + CO2
(MHCO3) 3. HNO3 + Zn(HCO3)2 → Zn(NO3)2 + H2O CO2
6. Non-Metallic 1. HCl + CO2 → No reaction.
Oxide (NMO) 2. H2SO4 + NO2 → No reaction.
2