Page 6 - LN
P. 6
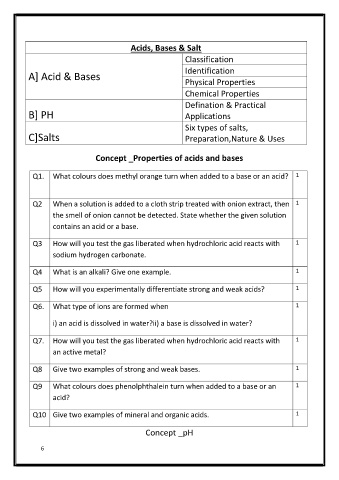
Acids, Bases & Salt
Classification
A] Acid & Bases Identification
Physical Properties
Chemical Properties
Defination & Practical
B] PH Applications
Six types of salts,
C]Salts Preparation,Nature & Uses
Concept _Properties of acids and bases
Q1. What colours does methyl orange turn when added to a base or an acid? 1
Q2 When a solution is added to a cloth strip treated with onion extract, then 1
the smell of onion cannot be detected. State whether the given solution
contains an acid or a base.
Q3 How will you test the gas liberated when hydrochloric acid reacts with 1
sodium hydrogen carbonate.
Q4 What is an alkali? Give one example. 1
Q5 How will you experimentally differentiate strong and weak acids? 1
Q6. What type of ions are formed when 1
i) an acid is dissolved in water?ii) a base is dissolved in water?
Q7. How will you test the gas liberated when hydrochloric acid reacts with 1
an active metal?
Q8 Give two examples of strong and weak bases. 1
Q9 What colours does phenolphthalein turn when added to a base or an 1
acid?
Q10 Give two examples of mineral and organic acids. 1
Concept _pH
6