Page 2 - LN
P. 2
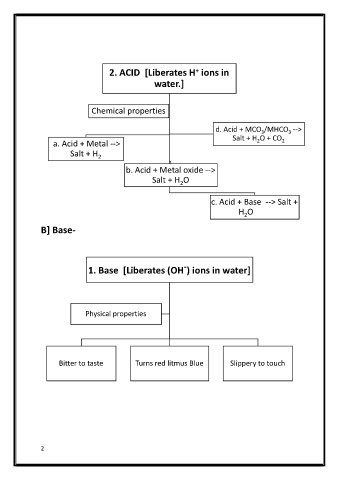
2. ACID [Liberates H ions in
+
water.]
Chemical properties
d. Acid + MCO /MHCO -->
3
3
Salt + H O + CO
a. Acid + Metal --> 2 2
Salt + H 2
b. Acid + Metal oxide -->
Salt + H O
2
c. Acid + Base --> Salt +
H O
2
B] Base-
-
1. Base [Liberates (OH ) ions in water]
Physical properties
Bitter to taste Turns red litmus Blue Slippery to touch
2