Page 13 - Lesson Notes - Biomolecules 1
P. 13
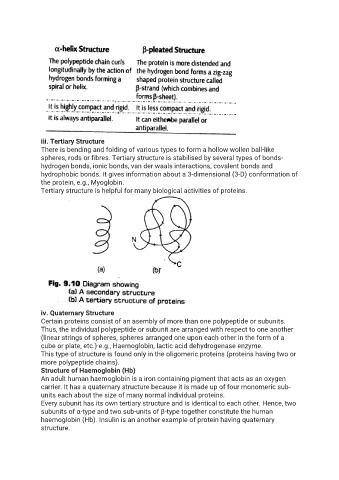
iii. Tertiary Structure
There is bending and folding of various types to form a hollow wollen ball-like
spheres, rods or fibres. Tertiary structure is stabilised by several types of bonds-
hydrogen bonds, ionic bonds, van der waals interactions, covalent bonds and
hydrophobic bonds. It gives information about a 3-dimensional (3-D) conformation of
the protein, e.g., Myoglobin.
Tertiary structure is helpful for many biological activities of proteins.
iv. Quaternary Structure
Certain proteins consist of an asembly of more than one polypeptide or subunits.
Thus, the individual polypeptide or subunit are arranged with respect to one another
(linear strings of spheres, spheres arranged one upon each other in the form of a
cube or plate, etc.) e.g., Haemoglobin, lactic acid dehydrogenase enzyme.
This type of structure is found only in the oligomeric proteins (proteins having two or
more polypeptide chains).
Structure of Haemoglobin (Hb)
An adult human haemoglobin is a iron containing pigment that acts as an oxygen
carrier. It has a quaternary structure because it is made up of four monomeric sub-
units each about the size of many normal individual proteins.
Every subunit has its own tertiary structure and is identical to each other. Hence, two
subunits of α-type and two sub-units of β-type together constitute the human
haemoglobin (Hb). Insulin is an another example of protein having quaternary
structure.