Page 5 - 1. MIND MAP- ( CH-3 FULL CHAPTER)
P. 5
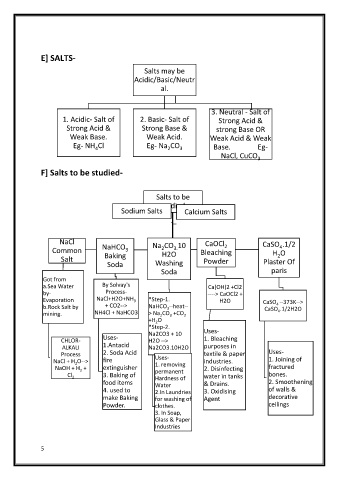
E] SALTS-
Salts may be
Acidic/Basic/Neutr
al.
3. Neutral - Salt of
1. Acidic- Salt of 2. Basic- Salt of Strong Acid &
Strong Acid & Strong Base & strong Base OR
Weak Base. Weak Acid.
Weak Acid & Weak
Eg- NH Cl Eg- Na CO 3 Base. Eg-
4
2
NaCl, CuCO 3
F] Salts to be studied-
Salts to be
studied
Sodium Salts Calcium Salts
NaCl Na CO 10 CaOCl CaSO .1/2
2
4
3.
Common NaHCO 3 2 H2O Bleaching H O
Salt Baking Powder 2
Soda Washing Plaster Of
Soda paris
Got from
a.Sea Water By Solvay's Ca(OH)2 +Cl2
by- Process- ----> CaOCl2 +
Evaporation NaCl+H2O+NH 3 *Step-1. H2O CaSO --373K-->
4
b.Rock Salt by + CO2--> NaHCO --heat-- CaSO .1/2H2O
3
mining. NH4Cl + NaHCO3 > Na CO +CO 2 4
3
2
+H O
2
*Step-2.
Na2CO3 + 10 Uses-
Uses- 1. Bleaching
CHLOR- H2O -->
ALKALI 1.Antacid Na2CO3.10H2O purposes in
2. Soda Acid
Process Uses- textile & paper Uses-
NaCl + H O--> fire industries. 1. Joining of
2
NaOH + H + extinguisher 1. removing 2. Disinfecting fractured
2
permanent
Cl 2 3. Baking of Hardness of water in tanks bones.
food items Water & Drains. 2. Smoothening
4. used to 2.In Laundries 3. Oxidising of walls &
make Baking for washing of Agent decorative
Powder. clothes. ceilings
3. In Soap,
Glass & Paper
Industries
5