Page 3 - 1. MIND MAP- ( CH-3 FULL CHAPTER)
P. 3
-
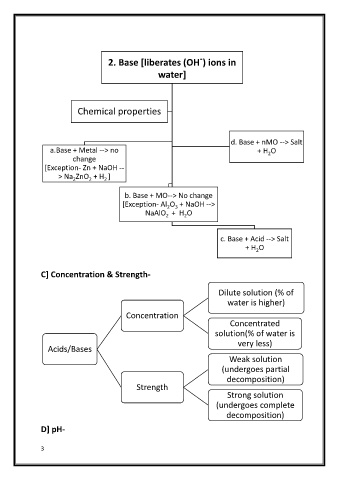
2. Base [liberates (OH ) ions in
water]
Chemical properties
d. Base + nMO --> Salt
a.Base + Metal --> no + H O
2
change
[Exception- Zn + NaOH --
> Na ZnO + H ]
2
2
2
b. Base + MO--> No change
[Exception- Al O + NaOH -->
2 3
NaAlO + H O
2
2
c. Base + Acid --> Salt
+ H O
2
C] Concentration & Strength-
Dilute solution (% of
water is higher)
Concentration
Concentrated
solution(% of water is
very less)
Acids/Bases
Weak solution
(undergoes partial
decomposition)
Strength
Strong solution
(undergoes complete
decomposition)
D] pH-
3