Page 2 - HA
P. 2
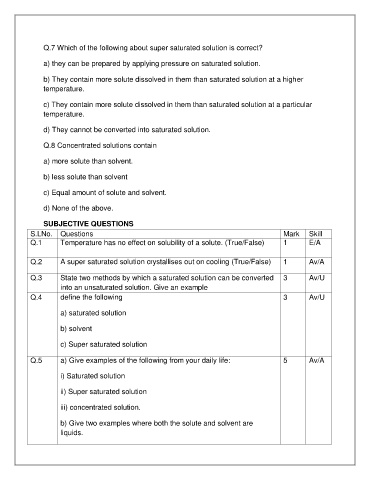
Q.7 Which of the following about super saturated solution is correct?
a) they can be prepared by applying pressure on saturated solution.
b) They contain more solute dissolved in them than saturated solution at a higher
temperature.
c) They contain more solute dissolved in them than saturated solution at a particular
temperature.
d) They cannot be converted into saturated solution.
Q.8 Concentrated solutions contain
a) more solute than solvent.
b) less solute than solvent
c) Equal amount of solute and solvent.
d) None of the above.
SUBJECTIVE QUESTIONS
S.LNo. Questions Mark Skill
Q.1 Temperature has no effect on solubility of a solute. (True/False) 1 E/A
Q.2 A super saturated solution crystallises out on cooling (True/False) 1 Av/A
Q.3 State two methods by which a saturated solution can be converted 3 Av/U
into an unsaturated solution. Give an example
Q.4 define the following 3 Av/U
a) saturated solution
b) solvent
c) Super saturated solution
Q.5 a) Give examples of the following from your daily life: 5 Av/A
i) Saturated solution
ii) Super saturated solution
iii) concentrated solution.
b) Give two examples where both the solute and solvent are
liquids.