Page 2 - LN
P. 2
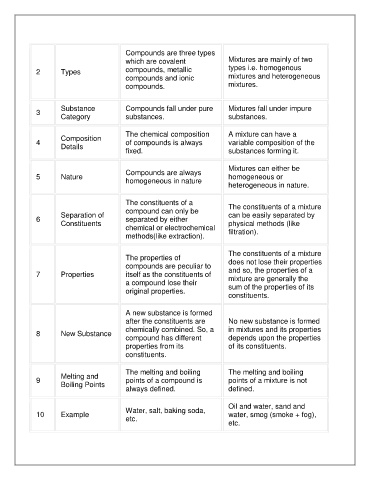
Compounds are three types
which are covalent Mixtures are mainly of two
2 Types compounds, metallic types i.e. homogenous
compounds and ionic mixtures and heterogeneous
compounds. mixtures.
Substance Compounds fall under pure Mixtures fall under impure
3
Category substances. substances.
The chemical composition A mixture can have a
Composition
4 of compounds is always variable composition of the
Details
fixed. substances forming it.
Mixtures can either be
Compounds are always
5 Nature homogeneous or
homogeneous in nature
heterogeneous in nature.
The constituents of a The constituents of a mixture
compound can only be
Separation of can be easily separated by
6 separated by either
Constituents physical methods (like
chemical or electrochemical
methods(like extraction). filtration).
The constituents of a mixture
The properties of does not lose their properties
compounds are peculiar to
7 Properties itself as the constituents of and so, the properties of a
mixture are generally the
a compound lose their
original properties. sum of the properties of its
constituents.
A new substance is formed
after the constituents are No new substance is formed
chemically combined. So, a in mixtures and its properties
8 New Substance
compound has different depends upon the properties
properties from its of its constituents.
constituents.
The melting and boiling The melting and boiling
Melting and
9 points of a compound is points of a mixture is not
Boiling Points
always defined. defined.
Oil and water, sand and
Water, salt, baking soda,
10 Example water, smog (smoke + fog),
etc.
etc.