Page 3 - HA
P. 3
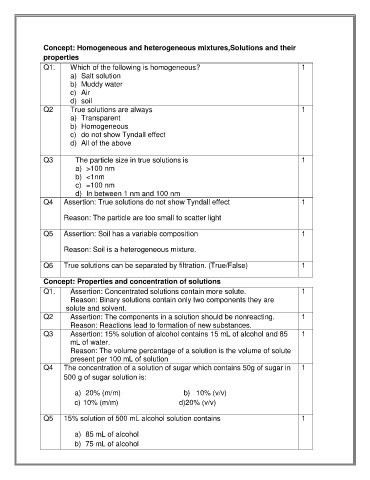
Concept: Homogeneous and heterogeneous mixtures,Solutions and their
properties
Q1. Which of the following is homogeneous? 1
a) Salt solution
b) Muddy water
c) Air
d) soil
Q2 True solutions are always 1
a) Transparent
b) Homogeneous
c) do not show Tyndall effect
d) All of the above
Q3 The particle size in true solutions is 1
a) >100 nm
b) <1nm
c) =100 nm
d) In between 1 nm and 100 nm
Q4 Assertion: True solutions do not show Tyndall effect 1
Reason: The particle are too small to scatter light
Q5 Assertion: Soil has a variable composition 1
Reason: Soil is a heterogeneous mixture.
Q6 True solutions can be separated by filtration. (True/False) 1
Concept: Properties and concentration of solutions
Q1. Assertion: Concentrated solutions contain more solute. 1
Reason: Binary solutions contain only two components they are
solute and solvent.
Q2 Assertion: The components in a solution should be nonreacting. 1
Reason: Reactions lead to formation of new substances.
Q3 Assertion: 15% solution of alcohol contains 15 mL of alcohol and 85 1
mL of water.
Reason: The volume percentage of a solution is the volume of solute
present per 100 mL of solution
Q4 The concentration of a solution of sugar which contains 50g of sugar in 1
500 g of sugar solution is:
a) 20% (m/m) b) 10% (v/v)
c) 10% (m/m) d)20% (v/v)
Q5 15% solution of 500 mL alcohol solution contains 1
a) 85 mL of alcohol
b) 75 mL of alcohol