Page 2 - 4.HOME ASSIGNMENT-(REDOX REACTIONS)
P. 2
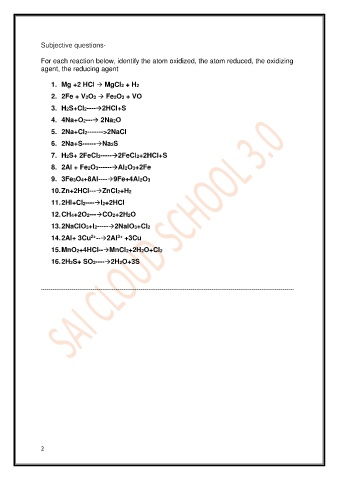
Subjective questions-
For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing
agent, the reducing agent
1. Mg +2 HCl → MgCl2 + H2
2. 2Fe + V2O3 → Fe2O3 + VO
3. H2S+Cl2----→2HCl+S
4. 4Na+O2---→ 2Na2O
5. 2Na+Cl2------->2NaCl
6. 2Na+S------→Na2S
7. H2S+ 2FeCl3-----→2FeCl2+2HCl+S
8. 2Al + Fe2O3------→Al2O3+2Fe
9. 3Fe3O4+8Al----→9Fe+4Al2O3
10. Zn+2HCl---→ZnCl2+H2
11. 2HI+Cl2----→I2+2HCl
12. CH4+2O2---→CO2+2H2O
13. 2NaClO3+I2-----→2NaIO3+Cl2
2+
3+
14. 2Al+ 3Cu --→2Al +3Cu
15. MnO2+4HCl--→MnCl2+2H2O+Cl2
16. 2H2S+ SO2----→2H2O+3S
------------------------------------------------------------------------------------------------------------------------------------
2