Page 2 - 3. LESSON NOTE-(COMBINATION & DECOMPOSITION REACTION)
P. 2
• Photo decomposition reaction.
Thermal decomposition reaction A thermal decomposition reaction can be defined as a
decomposition reaction which is activated by thermal energy. The reaction is generally
endothermic as energy is required to break chemical bond in order to separate the constituent
elements.
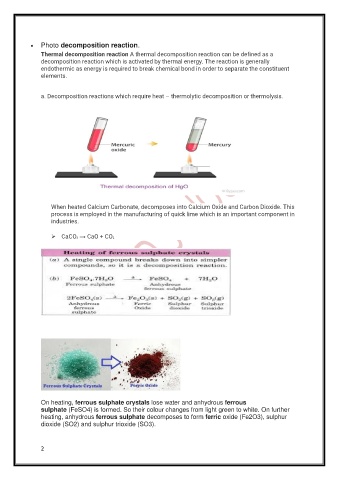
a. Decomposition reactions which require heat – thermolytic decomposition or thermolysis.
When heated Calcium Carbonate, decomposes into Calcium Oxide and Carbon Dioxide. This
process is employed in the manufacturing of quick lime which is an important component in
industries.
➢ CaCO3 → CaO + CO2
On heating, ferrous sulphate crystals lose water and anhydrous ferrous
sulphate (FeSO4) is formed. So their colour changes from light green to white. On further
heating, anhydrous ferrous sulphate decomposes to form ferric oxide (Fe2O3), sulphur
dioxide (SO2) and sulphur trioxide (SO3).
2