Page 2 - HA
P. 2
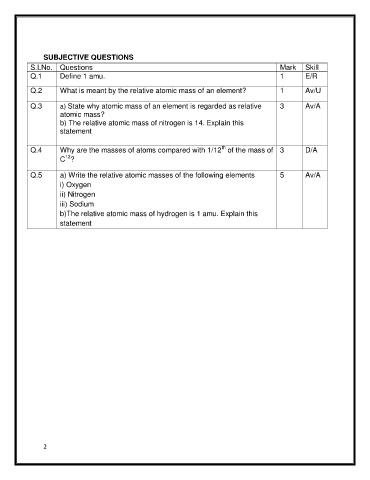
SUBJECTIVE QUESTIONS
S.LNo. Questions Mark Skill
Q.1 Define 1 amu. 1 E/R
Q.2 What is meant by the relative atomic mass of an element? 1 Av/U
Q.3 a) State why atomic mass of an element is regarded as relative 3 Av/A
atomic mass?
b) The relative atomic mass of nitrogen is 14. Explain this
statement
th
Q.4 Why are the masses of atoms compared with 1/12 of the mass of 3 D/A
12
C ?
Q.5 a) Write the relative atomic masses of the following elements 5 Av/A
i) Oxygen
ii) Nitrogen
iii) Sodium
b)The relative atomic mass of hydrogen is 1 amu. Explain this
statement
2