Page 2 - HA2
P. 2
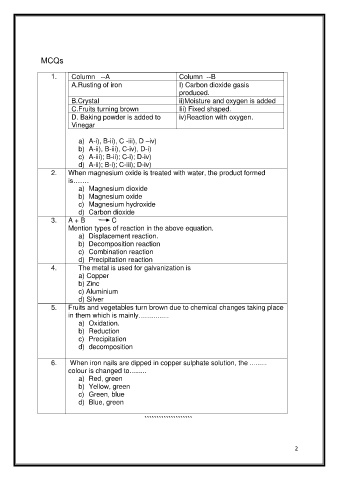
MCQs
1. Column --A Column --B
A.Rusting of iron I) Carbon dioxide gasis
produced.
B.Crystal ii)Moisture and oxygen is added
C.Fruits turning brown Iii) Fixed shaped.
D. Baking powder is added to iv)Reaction with oxygen.
Vinegar
a) A-i), B-ii), C -iii), D –iv)
b) A-ii), B-iii), C-iv), D-i)
c) A-iii); B-ii); C-i); D-iv)
d) A-ii); B-i); C-iii); D-iv)
2. When magnesium oxide is treated with water, the product formed
is…….
a) Magnesium dioxide
b) Magnesium oxide
c) Magnesium hydroxide
d) Carbon dioxide
3. A + B C
Mention types of reaction in the above equation.
a) Displacement reaction.
b) Decomposition reaction
c) Combination reaction
d) Precipitation reaction
4. The metal is used for galvanization is
a) Copper
b) Zinc
c) Aluminium
d) Silver
5. Fruits and vegetables turn brown due to chemical changes taking place
in them which is mainly................
a) Oxidation.
b) Reduction
c) Precipitation
d) decomposition
6. When iron nails are dipped in copper sulphate solution, the .........
colour is changed to.........
a) Red, green
b) Yellow, green
c) Green, blue
d) Blue, green
````````````````````
2