Page 2 - LN 8
P. 2
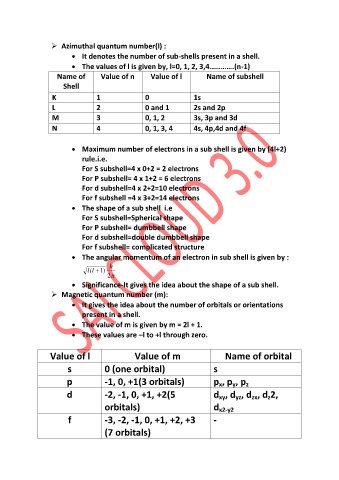
Azimuthal quantum number(l) :
It denotes the number of sub-shells present in a shell.
The values of l is given by, l=0, 1, 2, 3,4………….(n-1)
Name of Value of n Value of l Name of subshell
Shell
K 1 0 1s
L 2 0 and 1 2s and 2p
M 3 0, 1, 2 3s, 3p and 3d
N 4 0, 1, 3, 4 4s, 4p,4d and 4f
Maximum number of electrons in a sub shell is given by (4l+2)
rule.i.e.
For S subshell=4 x 0+2 = 2 electrons
For P subshell= 4 x 1+2 = 6 electrons
For d subshell=4 x 2+2=10 electrons
For f subshell =4 x 3+2=14 electrons
The shape of a sub shell i.e
For S subshell=Spherical shape
For P subshell= dumbbell shape
For d subshell=double dumbbell shape
For f subshell= complicated structure
The angular momentum of an electron in sub shell is given by :
h
(l l ) 1
2
Significance-It gives the idea about the shape of a sub shell.
Magnetic quantum number (m):
It gives the idea about the number of orbitals or orientations
present in a shell.
The value of m is given by m = 2l + 1.
These values are –l to +l through zero.
Value of l Value of m Name of orbital
s 0 (one orbital) s
p -1, 0, +1(3 orbitals) p , p , p
x
z
y
d -2, -1, 0, +1, +2(5 d , d , d , d 2,
xy
z
zx
yz
orbitals) d x2-y2
f -3, -2, -1, 0, +1, +2, +3 -
(7 orbitals)