Page 2 - LN 7
P. 2
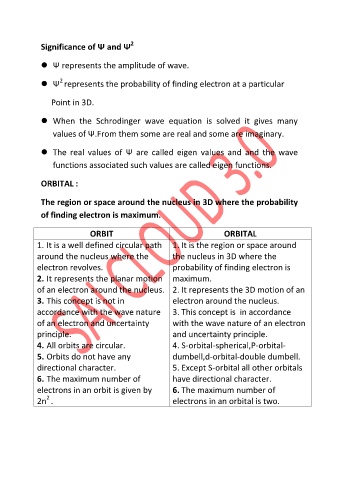
Significance of Ψ and Ψ 2
Ψ represents the amplitude of wave.
2
Ψ represents the probability of finding electron at a particular
Point in 3D.
When the Schrodinger wave equation is solved it gives many
values of Ψ.From them some are real and some are imaginary.
The real values of Ψ are called eigen values and and the wave
functions associated such values are called eigen functions.
ORBITAL :
The region or space around the nucleus in 3D where the probability
of finding electron is maximum.
ORBIT ORBITAL
1. It is a well defined circular path 1. It is the region or space around
around the nucleus where the the nucleus in 3D where the
electron revolves. probability of finding electron is
2. It represents the planar motion maximum.
of an electron around the nucleus. 2. It represents the 3D motion of an
3. This concept is not in electron around the nucleus.
accordance with the wave nature 3. This concept is in accordance
of an electron and uncertainty with the wave nature of an electron
principle. and uncertainty principle.
4. All orbits are circular. 4. S-orbital-spherical,P-orbital-
5. Orbits do not have any dumbell,d-orbital-double dumbell.
directional character. 5. Except S-orbital all other orbitals
6. The maximum number of have directional character.
electrons in an orbit is given by 6. The maximum number of
2
2n . electrons in an orbital is two.