Page 2 - LN 6
P. 2
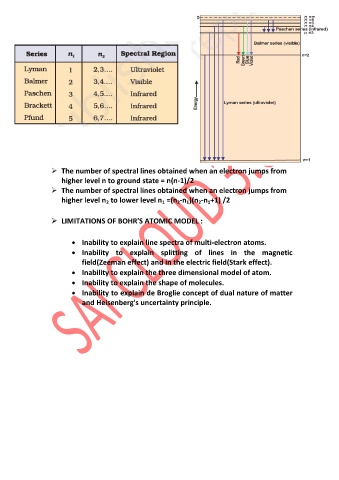
The number of spectral lines obtained when an electron jumps from
higher level n to ground state = n(n-1)/2
The number of spectral lines obtained when an electron jumps from
higher level n to lower level n =(n -n )(n -n +1) /2
1
2
1
2
2
1
LIMITATIONS OF BOHR'S ATOMIC MODEL :
Inability to explain line spectra of multi-electron atoms.
Inability to explain splitting of lines in the magnetic
field(Zeeman effect) and in the electric field(Stark effect).
Inability to explain the three dimensional model of atom.
Inability to explain the shape of molecules.
Inability to explain de Broglie concept of dual nature of matter
and Heisenberg's uncertainty principle.