Page 5 - LN
P. 5
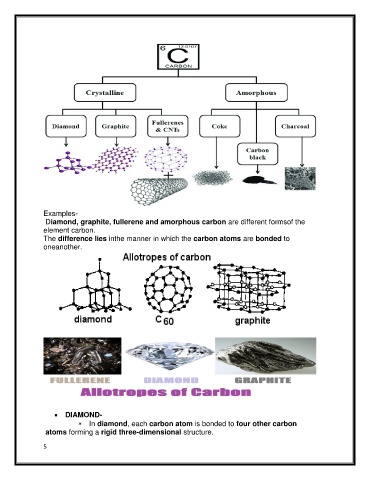
Examples-
Diamond, graphite, fullerene and amorphous carbon are different formsof the
element carbon.
The difference lies inthe manner in which the carbon atoms are bonded to
oneanother.
DIAMOND-
In diamond, each carbon atom is bonded to four other carbon
atoms forming a rigid three-dimensional structure.
5