Page 2 - HA-3
P. 2
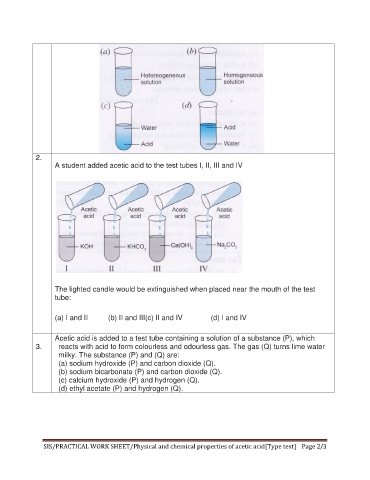
2.
A student added acetic acid to the test tubes I, II, III and IV
The lighted candle would be extinguished when placed near the mouth of the test
tube:
(a) I and II (b) II and III(c) II and IV (d) I and IV
Acetic acid is added to a test tube containing a solution of a substance (P), which
3. reacts with acid to form colourless and odourless gas. The gas (Q) turns lime water
milky. The substance (P) and (Q) are:
(a) sodium hydroxide (P) and carbon dioxide (Q).
(b) sodium bicarbonate (P) and carbon dioxide (Q).
(c) calcium hydroxide (P) and hydrogen (Q).
(d) ethyl acetate (P) and hydrogen (Q).
SIS/PRACTICAL WORK SHEET/Physical and chemical properties of acetic acid[Type text] Page 2/3