Page 1 - 2. Lesson note - Ch-3 Concept mapping
P. 1
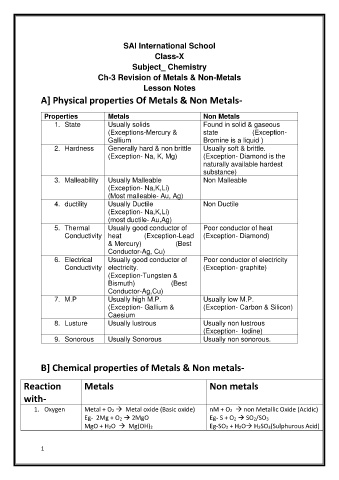
SAI International School
Class-X
Subject_ Chemistry
Ch-3 Revision of Metals & Non-Metals
Lesson Notes
A] Physical properties Of Metals & Non Metals-
Properties Metals Non Metals
1. State Usually solids in solid & gaseous
u
n
d
Fo
(Exceptions-Mercury & state (Exception-
Gallium Bromine is a liquid )
2. Hardness Generally hard & non brittle Usually soft & brittle.
(Exception- Na, K, Mg) (Exception- Diamond is the
naturally available hardest
substance)
3. Malleability Usually Malleable Non Malleable
(Exception- Na,K,Li)
(Most malleable- Au, Ag)
Non
4. ductility Usually Ductile ctile
Du
(Exception- Na,K,Li)
(most ductile- Au,Ag)
5. Thermal Usually good conductor of Poor conductor of heat
Conductivity heat (Exception-Lead (Exception- Diamond)
& Mercury) (Best
Conductor-Ag, Cu)
6. Electrical Usually good conductor of Poor conductor of electricity
Conductivity electricity. (Exception- graphite)
(Exception-Tungsten &
Bismuth) (Best
Conductor-Ag,Cu)
Usua
ll
7. M.P Usually high M.P. y low M.P.
(Exception- Gallium & (Exception- Carbon & Silicon)
Caesium
8. Lusture Usually lustrous Usually non lustrous
(Exception- Iodine)
9. Sonorous Usually Sonorous Usually non sonorous.
B] Chemical properties of Metals & Non metals-
Reaction Metals Non metals
with-
1. Oxygen Metal + O2 → Metal oxide (Basic oxide) M + O2 → non Metallic Oxide (Acidic)
n
Eg
Eg- 2Mg + O2 → 2MgO - S + O2 → SO2/SO3
MgO + H2O → Mg(OH)2 Eg-SO2 + H2O→ H2SO3(Sulphurous Acid)
1