Page 2 - 4. Home Assignment-Ch-3 Covalent Bonding
P. 2
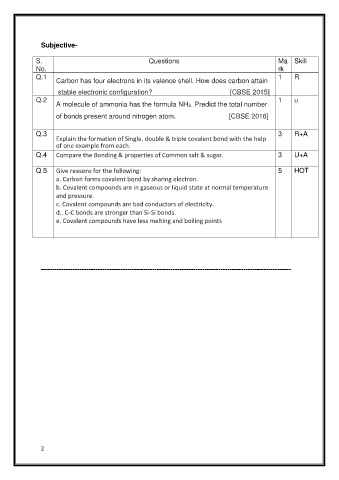
Subjective-
S.L Questions Ma Skill
No. rk
Q.1 1 R
Carbon has four electrons in its valence shell. How does carbon attain
stable electronic configuration? [CBSE 2015]
Q.2 1 u
A molecule of ammonia has the formula NH3. Predict the total number
of bonds present around nitrogen atom. [CBSE 2016]
Q.3 3 R+A
Explain the formation of Single, double & triple covalent bond with the help
of one example from each.
Q.4 Compare the Bonding & properties of Common salt & sugar. 3 U+A
Q.5 Give reasons for the following: 5 HOT
a. Carbon forms covalent bond by sharing electron.
b. Covalent compounds are in gaseous or liquid state at normal temperature
and pressure.
c. Covalent compounds are bad conductors of electricity.
d.. C-C bonds are stronger than Si-Si bonds.
e. Covalent compounds have less melting and boiling points
--------------------------------------------------------------------------------------------------------------
2