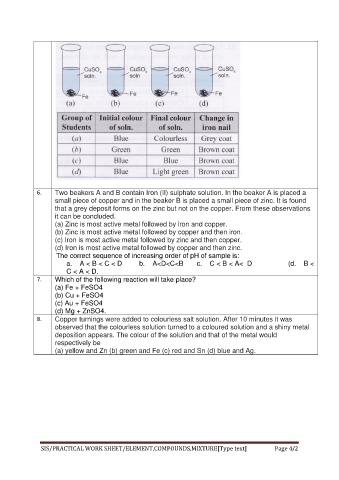
Page 4 - 2. HA (Lab WS_Expt_4)
P. 4
6. Two beakers A and B contain Iron (II) sulphate solution. In the beaker A is placed a
small piece of copper and in the beaker B is placed a small piece of zinc. It is found
that a grey deposit forms on the zinc but not on the copper. From these observations
it can be concluded.
(a) Zinc is most active metal followed by iron and copper.
(b) Zinc is most active metal followed by copper and then iron.
(c) Iron is most active metal followed by zinc and then copper.
(d) Iron is most active metal followed by copper and then zinc.
The correct sequence of increasing order of pH of sample is:
a. A < B < C < D b. A<D<C<B c. C < B < A< D (d. B <
C < A < D.
7. Which of the following reaction will take place?
(a) Fe + FeSO4
(b) Cu + FeSO4
(c) Au + FeSO4
(d) Mg + ZnSO4.
8. Copper turnings were added to colourless salt solution. After 10 minutes it was
observed that the colourless solution turned to a coloured solution and a shiny metal
deposition appears. The colour of the solution and that of the metal would
respectively be
(a) yellow and Zn (b) green and Fe (c) red and Sn (d) blue and Ag.
SIS/PRACTICAL WORK SHEET/ELEMENT,COMPOUNDS,MIXTURE[Type text] Page 4/2