Page 2 - LN
P. 2
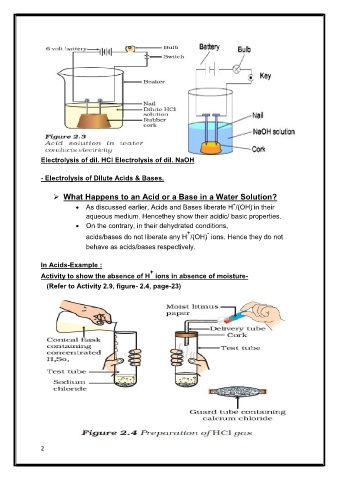
Electrolysis of dil. HCl Electrolysis of dil. NaOH
- Electrolysis of Dilute Acids & Bases.
What Happens to an Acid or a Base in a Water Solution?
+
-
As discussed earlier, Acids and Bases liberate H /(OH) in their
aqueous medium. Hencethey show their acidic/ basic properties.
On the contrary, in their dehydrated conditions,
+ -
acids/bases do not liberate any H /(OH) ions. Hence they do not
behave as acids/bases respectively.
In Acids-Example :
+
Activity to show the absence of H ions in absence of moisture-
(Refer to Activity 2.9, figure- 2.4, page-23)
2