Page 2 - HA
P. 2
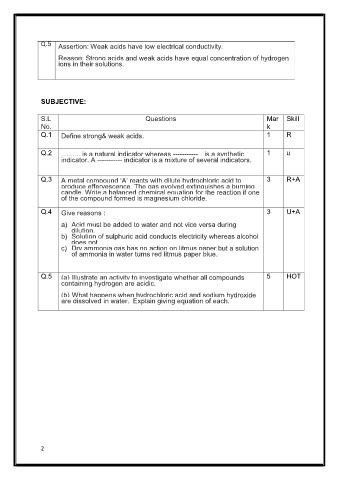
Q.5 Assertion: Weak acids have low electrical conductivity.
Reason: Strong acids and weak acids have equal concentration of hydrogen
ions in their solutions.
SUBJECTIVE:
S.L Questions Mar Skill
No. k
Q.1 Define strong& weak acids. 1 R
Q.2 .......... is a natural indicator whereas ----------- is a synthetic 1 u
indicator. A ----------- indicator is a mixture of several indicators.
Q.3 A metal compound ‘A’ reacts with dilute hydrochloric acid to 3 R+A
produce effervescence. The gas evolved extinguishes a burning
candle. Write a balanced chemical equation for the reaction if one
of the compound formed is magnesium chloride.
Q.4 Give reasons : 3 U+A
a) Acid must be added to water and not vice versa during
dilution.
b) Solution of sulphuric acid conducts electricity whereas alcohol
does not .
c) Dry ammonia gas has no action on litmus paper but a solution
of ammonia in water turns red litmus paper blue.
Q.5 (a) Illustrate an activity to investigate whether all compounds 5 HOT
containing hydrogen are acidic.
(b) What happens when hydrochloric acid and sodium hydroxide
are dissolved in water. Explain giving equation of each.
2