Page 2 - HA
P. 2
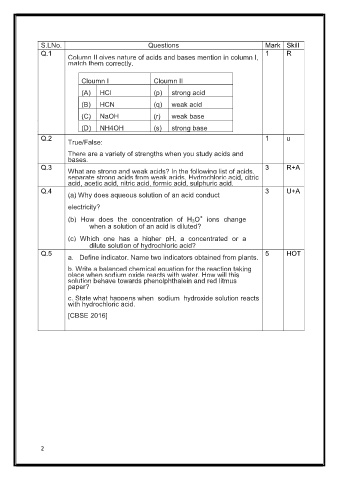
S.LNo. Questions Mark Skill
Q.1 1 R
Column II gives nature of acids and bases mention in column I,
match them correctly.
Cloumn I Cloumn II
(A) HCl (p) strong acid
(B) HCN (q) weak acid
(C) NaOH (r) weak base
(D) NH4OH (s) strong base
Q.2 1 u
True/False:
There are a variety of strengths when you study acids and
bases.
Q.3 3 R+A
What are strong and weak acids? In the following list of acids,
separate strong acids from weak acids. Hydrochloric acid, citric
acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Q.4 3 U+A
(a) Why does aqueous solution of an acid conduct
electricity?
+
(b) How does the concentration of H 3O ions change
when a solution of an acid is diluted?
(c) Which one has a higher pH, a concentrated or a
dilute solution of hydrochloric acid?
Q.5 5 HOT
a. Define indicator. Name two indicators obtained from plants.
b. Write a balanced chemical equation for the reaction taking
place when sodium oxide reacts with water. How will this
solution behave towards phenolphthalein and red litmus
paper?
c. State what happens when sodium hydroxide solution reacts
with hydrochloric acid.
[CBSE 2016]
2