Page 2 - LN
P. 2
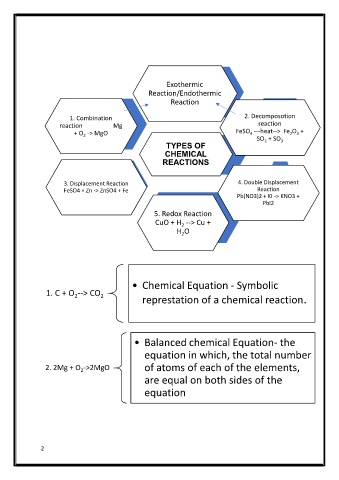
Exothermic
Reaction/Endothermic
Exothermic Reaction
Reaction
1. Combination 2. Decomposotion
Pb(NO3)2 + KI -> KNO3 + PbI2
Double Displacement Reaction
reaction Mg reaction
Combination reaction Mg + O 2 -> MgO
+ O -> MgO FeSO ---heat--> Fe O +
4
2 3
2
SO + SO
TYPES OF
TYPES OF 2 3
CHEMICAL
CHEMICAL
REACTIONS
REACTIONS
Decomposotion
3. Displacement Reaction 4. Double Displacement
reaction
Displacement Reaction FeSO4 + Zn -> ZnSO4 + Fe
FeSO 4 ---heat-->
FeSO4 + Zn -> ZnSO4 + Fe Reaction
Fe 2 O 3 + SO 2 +
Pb(NO3)2 + KI -> KNO3 +
SO 3
PbI2
5. Redox Reaction
Endothermic Reaction
CuO + H --> Cu +
2
H O
2
• Chemical Equation - Symbolic
1. C + O --> CO 2 represtation of a chemical reaction.
2
• Balanced chemical Equation- the
equation in which, the total number
2. 2Mg + O ->2MgO of atoms of each of the elements,
2
are equal on both sides of the
equation
2