Page 17 - PPT physical and chemical changes
P. 17
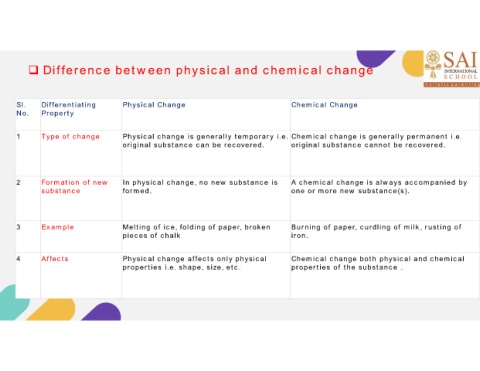
Difference between physical and chemical change
Sl. Differentiating Physical Change Chemical Change
No. Property
1 Type of change Physical change is generally temporary i.e. Chemical change is generally permanent i.e.
original substance can be recovered. original substance cannot be recovered.
2 Formation of new In physical change, no new substance is A chemical change is always accompanied by
substance formed. one or more new substance(s).
3 Example Melting of ice, folding of paper, broken Burning of paper, curdling of milk, rusting of
pieces of chalk iron.
4 Affects Physical change affects only physical Chemical change both physical and chemical
properties i.e. shape, size, etc. properties of the substance .