Page 1 - 1. MIND MAP-(ISOTOPES AND ISOBARS)
P. 1
SAI International School
Class-IX
Subject- Chemistry
Topic-Structure of atom
Concept Map
Lesson Notes
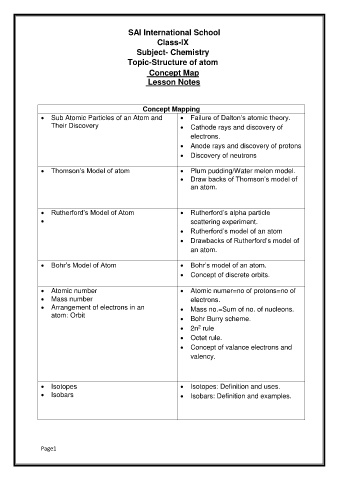
Concept Mapping
Sub Atomic Particles of an Atom and Failure of Dalton’s atomic theory.
Their Discovery Cathode rays and discovery of
electrons.
Anode rays and discovery of protons
Discovery of neutrons
Thomson’s Model of atom Plum pudding/Water melon model.
Draw backs of Thomson’s model of
an atom.
Rutherford’s Model of Atom Rutherford’s alpha particle
scattering experiment.
Rutherford’s model of an atom
Drawbacks of Rutherford’s model of
an atom.
Bohr’s Model of Atom Bohr’s model of an atom.
Concept of discrete orbits.
Atomic number Atomic numer=no of protons=no of
Mass number electrons.
Arrangement of electrons in an Mass no.=Sum of no. of nucleons.
atom: Orbit Bohr Burry scheme.
2n rule
2
Octet rule.
Concept of valance electrons and
valency.
Isotopes Isotopes: Definition and uses.
Isobars Isobars: Definition and examples.
Page1