Page 2 - 3. LESSON NOTES-(ISOTOPES AND ISOBARS)
P. 2
What are Isobars?
Isobar is that element which differs in the chemical property but has the same
physical property. So, we can say that isobars are those elements which have a
different atomic number but the same mass number. Their chemical property is
different because there is a difference in the number of electrons. It has the
same atomic mass but different atomic no. Because an additional number of
neutrons compensates the difference in the number of nucleons.
The example of two Isotopes and Isobars is Iron and Nickel. Both have the same
mass number which is 58 whereas the atomic number of iron is 26, and the atomic
number of nickel is 28.
Examples of Isobars:
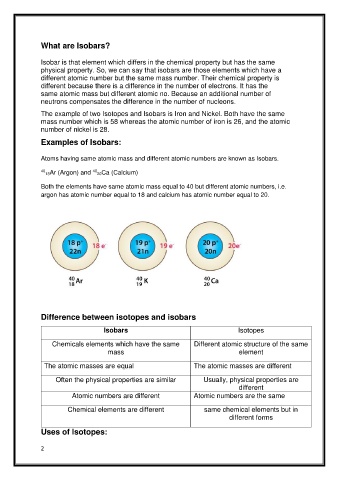
Atoms having same atomic mass and different atomic numbers are known as Isobars.
40 18Ar (Argon) and 40 20Ca (Calcium)
Both the elements have same atomic mass equal to 40 but different atomic numbers, i.e.
argon has atomic number equal to 18 and calcium has atomic number equal to 20.
Difference between isotopes and isobars
Isobars Isotopes
Chemicals elements which have the same Different atomic structure of the same
mass element
The atomic masses are equal The atomic masses are different
Often the physical properties are similar Usually, physical properties are
different
Atomic numbers are different Atomic numbers are the same
Chemical elements are different same chemical elements but in
different forms
Uses of Isotopes:
2