Page 2 - 3. LESSON NOTES-(BOHR BURRY SCHEME,DISTRIBUTION OF ELECTRONS IN VARIOUS SHELLS)
P. 2
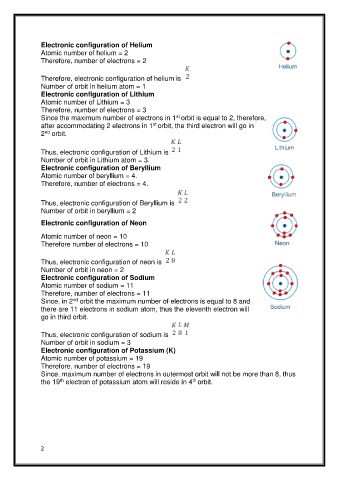
Electronic configuration of Helium
Atomic number of helium = 2
Therefore, number of electrons = 2
Therefore, electronic configuration of helium is
Number of orbit in helium atom = 1
Electronic configuration of Lithium
Atomic number of Lithium = 3
Therefore, number of electrons = 3
st
Since the maximum number of electrons in 1 orbit is equal to 2, therefore,
st
after accommodating 2 electrons in 1 orbit, the third electron will go in
2 orbit.
nd
Thus, electronic configuration of Lithium is
Number of orbit in Lithium atom = 3.
Electronic configuration of Beryllium
Atomic number of beryllium = 4.
Therefore, number of electrons = 4.
Thus, electronic configuration of Beryllium is
Number of orbit in beryllium = 2
Electronic configuration of Neon
Atomic number of neon = 10
Therefore number of electrons = 10
Thus, electronic configuration of neon is
Number of orbit in neon = 2
Electronic configuration of Sodium
Atomic number of sodium = 11
Therefore, number of electrons = 11
nd
Since, in 2 orbit the maximum number of electrons is equal to 8 and
there are 11 electrons in sodium atom, thus the eleventh electron will
go in third orbit.
Thus, electronic configuration of sodium is
Number of orbit in sodium = 3
Electronic configuration of Potassium (K)
Atomic number of potassium = 19
Therefore, number of electrons = 19
Since, maximum number of electrons in outermost orbit will not be more than 8, thus
th
the 19 electron of potassium atom will reside in 4 orbit.
th
2