Page 2 - PRACTICE WORKSHEET
P. 2
Q.7. In the atom of an element X, 6 electrons are present in the outermost shell. If it
acquires noble gas configuration by accepting requisite number of electrons, then what
would be the charge on the ion so formed?
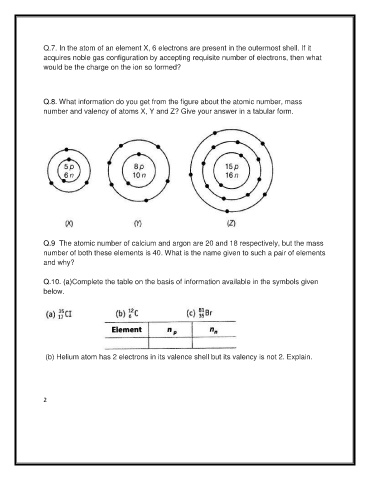
Q.8. What information do you get from the figure about the atomic number, mass
number and valency of atoms X, Y and Z? Give your answer in a tabular form.
Q.9 The atomic number of calcium and argon are 20 and 18 respectively, but the mass
number of both these elements is 40. What is the name given to such a pair of elements
and why?
Q.10. (a)Complete the table on the basis of information available in the symbols given
below.
(b) Helium atom has 2 electrons in its valence shell but its valency is not 2. Explain.
2